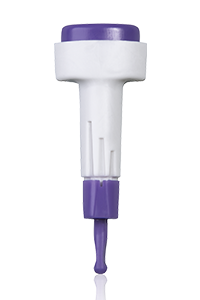

Multiple safety features combined with customized settings make the Accu-Chek
Safe-T-Pro Plus lancing device especially advantageous. This single use lancing device supports patient safety and ease of use. It also complies with the safety requirements demanded by health authorities, legislators, and health institutions.
Intended use
Single use, obtaining capillary blood from the fingertip or, if the patient is a child under 1 year from the heel.
Disposal
Entire device is for single use only.
Disposal of used single-use lancing devices always in accordance with the local / facility’s disposal procedures and country’s sanitary regulations.
In 3 milliseconds
1.3, 1.8 and 2.3 millimeters
4 years after sterilization
Entire device is for single use only.
always in accordance with the local / facility’s disposal procedures
Single use, obtaining capillary blood from the fingertip or, if the patient is a child under 1 year, from the heel.
- 3 adjustable depth settings allow the sampling of blood according to the skin condition of the patient
- Each depth setting clicks into place and cannot slip out of position once selected
- Special beveled needle cut and needle diameter for virtually pain-free blood sampling
- Use with adults, children under 1 year and neonates
- Save space and costs by using one type of lancet
- Designed for single use, to avoid cross contamination
- The Safe-T-technology ensures that the needle is always safely stored in the housing, preventing accidental needle sticks
- Automatic needle retraction after single use eliminates risk of injury and cross contamination
- After the single use the complete lancing device is disposed of
Single use only
Yes
Low (short marking) – 1.3mm
Medium (middle marking) – 1.8mm
High (long marking) – 2.3mm
3-facet cut
Yes
0.63 mm (23 G)
Yes
Yes
Yes
4 years after sterilization
Simple, 3-step testing:
- Twist the sterility cap and remove it.
- The penetration depth adjuster is pre-set at medium. Turn the penetration depth adjuster to the desired penetration depth. The 3 penetration depths are easy to identify by their short, medium, or long markings.
- Hold the lancing device between your index finger, middle finger, and thumb. You will find that it fits comfortably and securely between fingers, right or left-handed. Press the lancing device firmly against the chosen puncture site. Using your thumb, press the release button down completely. Move the lancing device away from the puncture site and discard it.
